Your Work done by gas formula images are ready. Work done by gas formula are a topic that is being searched for and liked by netizens today. You can Get the Work done by gas formula files here. Find and Download all free photos and vectors.
If you’re looking for work done by gas formula pictures information connected with to the work done by gas formula interest, you have pay a visit to the ideal blog. Our site always provides you with suggestions for seeking the highest quality video and picture content, please kindly hunt and find more informative video content and graphics that match your interests.
Work Done By Gas Formula. We can do a quick units check to see that pressure force area times volume gives units of force times length which are the units of work Joules or foot-pounds. For general cases we have to employ the integral texWork int P dVtex with the appropriate boundaries. W F s P A s and the area multiplied by the distance is a volume specifically the change in volume of the gas. The work done by the piston on the outside is.
 Work Done In Isothermal Process In Thermodynamics In Hindi Youtube From youtube.com
Work Done In Isothermal Process In Thermodynamics In Hindi Youtube From youtube.com
Whether the area is positive or negative depends on whether the gas expands or is. The work done by one mole of an ideal gas in the reversible process. The pressure of the surroundings c. We can use the definition of the work done by an ideal gas substitute the pressure by a function of the volume and use the givens for the volumes to calculate the work. What must remain constant for this equation to be used. Adiabatic heating occurs when the pressure of a gas is increased by work done on it by its surroundings eg.
The pressure of the surroundings c.
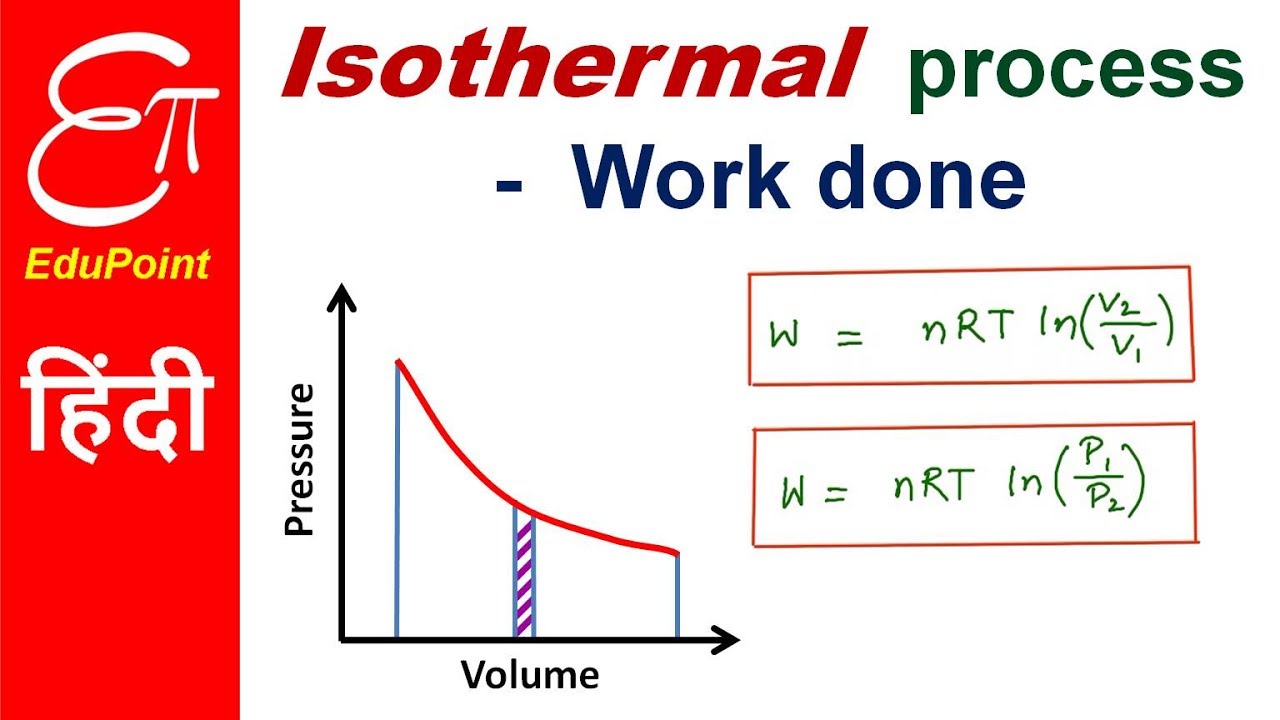
Adiabatic heating occurs when the pressure of a gas is increased by work done on it by its surroundings eg. The work done W by an expanding gas is calculated using W pΔV. Thus work done by the gas in an isothermal process is given by the expression W 2303RTlog 10dfracP_1P_2. The temperature of the surroundings e. EqW -nRTln frac V 2 V_ 1 eq Here W is the work done by the gas n is the number of moles. If work is done on the gas W.
 Source: in.pinterest.com
Source: in.pinterest.com
Adiabatic heating occurs when the pressure of a gas is increased by work done on it by its surroundings eg. The temperature of the surroundings e. Then the force acting on the piston F PA and small work done by the gas dW F dx PA dx p dV Since dW P dV a Graphical Method This case is different from the case of isothermal change in two respects. P V R T. When the force and the displacement are.
 Source: pinterest.com
Source: pinterest.com
Whether the area is positive or negative depends on whether the gas expands or is. Here expansion is caused due to internal pressure of gas against external pressure and so work is done please clarify me irreversible process formula endgroup. Work done W is defined as W -pdV. What must remain constant for this equation to be used. The work done by the piston on the outside is.
 Source: in.pinterest.com
Source: in.pinterest.com
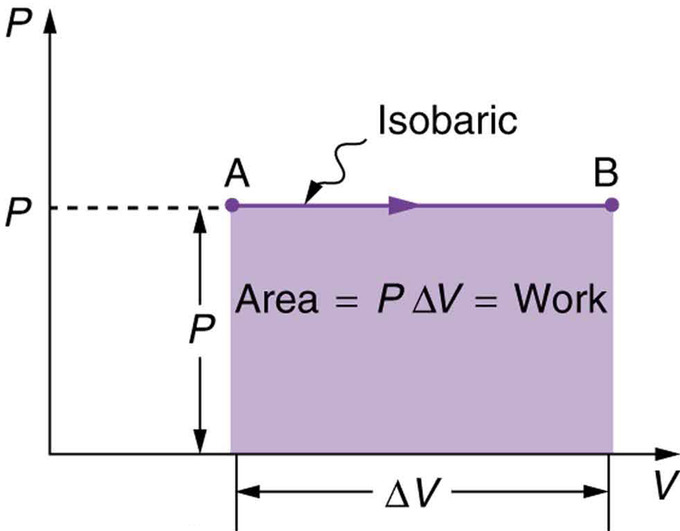
Work Done in Basic Thermodynamic Processes. The work done by the gas can be determined by working out the force applied by the gas and calculating the distance. Therefore P R T V. The work done when a volume of gas changes at constant pressure is defined as. The work done W by an expanding gas is calculated using W pΔV.
 Source: cuemath.com
Source: cuemath.com
The work done by the gas is represented on the P-V diagram by the rectangular area under the isobaric path on the diagram. This is just a mild rephrasing of the definition of work. Derivation of discrete formula and work expression. Adiabatic heating occurs when the pressure of a gas is increased by work done on it by its surroundings eg. What must remain constant for this equation to be used.
 Source: pinterest.com
Source: pinterest.com
The temperature of the expanding gas d. We can do a quick units check to see that pressure force area times volume gives units of force times length which are the units of work Joules or foot-pounds. P V R T. The SI unit for Gas pressure is expressed in Pascals Pa. Because dV 0 the work done is dW - P dV 0.
 Source: pinterest.com
Source: pinterest.com
The pressure of the surroundings c. Therefore P R T V. The work done by the piston on the outside is. For a small expansion of the gas pressure remains practically constant say P. The work is the blue hatched area under the curve which represents the process between state.
 Source: ohio.edu
Source: ohio.edu
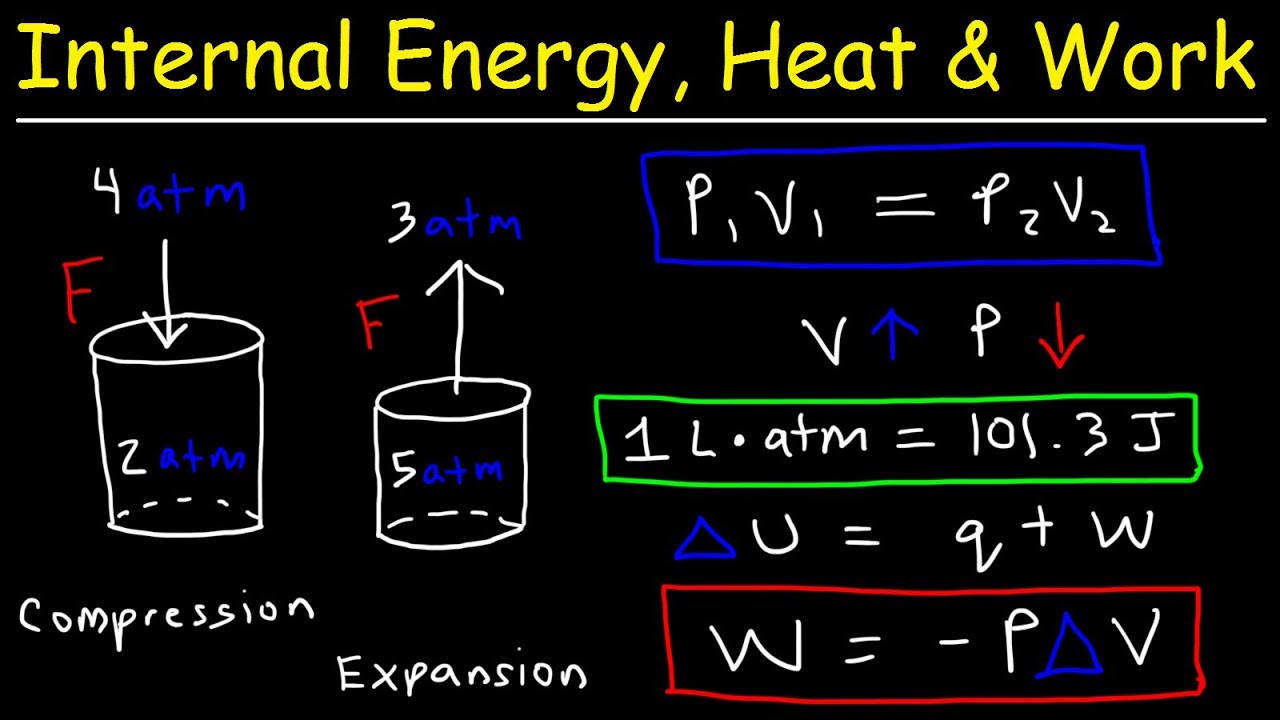
W work done J p external pressure Pa V volume of gas m 3 For a gas inside a cylinder enclosed by a moveable piston the force exerted by the gas pushes the piston outwards. We can use the definition of the work done by an ideal gas substitute the pressure by a function of the volume and use the givens for the volumes to calculate the work. The work is the blue hatched area under the curve which represents the process between state. The work done by the gas in an infinitesimal step is equal to the pressure multiplied by the change in volume. W nR 1γ T 1 T 2 W n R 1 γ T 1 T 2 In and adiabatic process if W0 ie work is done by the gas then T 2 T 1.
 Source: pinterest.com
Source: pinterest.com
For general cases we have to employ the integral texWork int P dVtex with the appropriate boundaries. EqW -nRTln frac V_ 2 V_ 1 eq Here W is the work done by the gas n is the number of moles. So work done on the gas depends upon the path. Hence by substituting the value of P in e q u a t. For general cases we have to employ the integral texWork int P dVtex with the appropriate boundaries.
 Source: pinterest.com
Source: pinterest.com
EqW -nRTln frac V_ 2 V_ 1 eq Here W is the work done by the gas n is the number of moles. A and b are correct Homework Equations W pΔV The Attempt at a Solution. Therefore P R T V. Thus work done by the gas in an isothermal process is given by the expression W 2303RTlog _10dfracP_1P_2. If work is done on the gas W.
 Source: pinterest.com
Source: pinterest.com
Thus work done by the gas in an isothermal process is given by the expression W 2303RTlog _10dfracP_1P_2. The change in internal energy of a system measured from state 1 to state 2 is equal to At the same time the work done by the pressurevolume changes as a result from this process is equal to. Because dV 0 the work done is dW - P dV 0. So at constant pressure work is just the pressure multiplied by the change. The work done when a volume of gas changes at constant pressure is defined as.
 Source: youtube.com
Source: youtube.com
For a gas work is the product of the pressure p and the volume V during a change of volume. The work done by the gas on the piston is W_1 int P_textint dV where P_textint is the pressure of the gas right next to the piston. When the force and the displacement are. When the gas is compressed V f V i V_f. The SI unit for Gas pressure is expressed in Pascals Pa.
 Source: ohio.edu
Source: ohio.edu
Here expansion is caused due to internal pressure of gas against external pressure and so work is done please clarify me irreversible process formula endgroup. Delta W p delta V The delta indicates a change in the variable. The Ideal gas pressure formula is given as Where V volume n number of moles R Gas constant 83145 JolmolK T temperature. The Gas pressure formula is given as Where F impact force due to gas collisions in Newtons N A area in meter square. Begingroup in irreversible isothermal expansion formula for work done is WPexternalx change in volume.
 Source: in.pinterest.com
Source: in.pinterest.com
For a small expansion of the gas pressure remains practically constant say P. So as we know an isothermal process is the one in which the pressure and volume of the gas changes at constant temperature. So work done on the gas depends upon the path. When the force and the displacement are. Whether the area is positive or negative depends on whether the gas expands or is.
 Source: wright.nasa.gov
Source: wright.nasa.gov
The work done W by an expanding gas is calculated using W pΔV. When work is done on the gas the volume of the gas decreases and work is positive. Work done W is defined as W -pdV. Hence by substituting the value of P in e q u a t. Derivation of discrete formula and work expression.
 Source: in.pinterest.com
Source: in.pinterest.com
Then the force acting on the piston F PA and small work done by the gas dW F dx PA dx p dV Since dW P dV a Graphical Method This case is different from the case of isothermal change in two respects. The work done by one mole of an ideal gas in the reversible process. Work is a path function. P V 3 constant from 1 a t m 3 0 0 K to 2 2 a t m is. Therefore P R T V.
 Source: pinterest.com
Source: pinterest.com
Adiabatic heating occurs when the pressure of a gas is increased by work done on it by its surroundings eg. P V R T. EqW -nRTln frac V_ 2 V_ 1 eq Here W is the work done by the gas n is the number of moles. W nR 1γ T 1 T 2 W n R 1 γ T 1 T 2 In and adiabatic process if W0 ie work is done by the gas then T 2 T 1. P V 3 constant from 1 a t m 3 0 0 K to 2 2 a t m is.
 Source: youtube.com
Source: youtube.com
Adiabatic heating occurs when the pressure of a gas is increased by work done on it by its surroundings eg. Hence Δ T 0. The temperature of the surroundings e. When the gas is compressed V f V i V_f. Then the force acting on the piston F PA and small work done by the gas dW F dx PA dx p dV Since dW P dV a Graphical Method This case is different from the case of isothermal change in two respects.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The equation texWork P Delta Vtex is true only for constant pressure. By ideal gas law. We can use the definition of the work done by an ideal gas substitute the pressure by a function of the volume and use the givens for the volumes to calculate the work. When the gas is compressed V f V i V_f. The pressure of the expanding gas b.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title work done by gas formula by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






