Your How to work out specific heat capacity images are ready. How to work out specific heat capacity are a topic that is being searched for and liked by netizens now. You can Get the How to work out specific heat capacity files here. Find and Download all free images.
If you’re searching for how to work out specific heat capacity pictures information linked to the how to work out specific heat capacity keyword, you have come to the ideal site. Our site always gives you suggestions for refferencing the maximum quality video and picture content, please kindly surf and locate more informative video content and graphics that match your interests.
How To Work Out Specific Heat Capacity. Specific Heat Capacity Formula Q C m t Where Q quantity of heat absorbed by a body m mass of the body t Rise in temperature C Specific heat capacity of a substance depends on the nature of the material of the substance. C Q m x ΔT Q supplied or subtracted heat in joules M the samples mass ΔT initial and final temperature difference JkgK heat capacity measurement type Specific Heat Values The heat capacity calculator is going to be useful in many situations. How to calculate specific heat capacity. C 196876125100 c 1575 Jg 0 C.
 Specific Heat Capacity Physics Lessons Science Facts Science Teaching Resources From in.pinterest.com
Specific Heat Capacity Physics Lessons Science Facts Science Teaching Resources From in.pinterest.com
Specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius. C 196876125100 c 1575 Jg 0 C. The new heat capacity depends on the. C specific heat capacity Jkg-1K-1 θ temperature change. Divide the heat. Find the final temperature of the mixture if two cup of water having masses m1150g and m2250g and temperatures T1 30 ºC and T275.
Change in thermal energy mass specific heat capacity temperature change Delta E_ t m times c times Delta theta This is when.
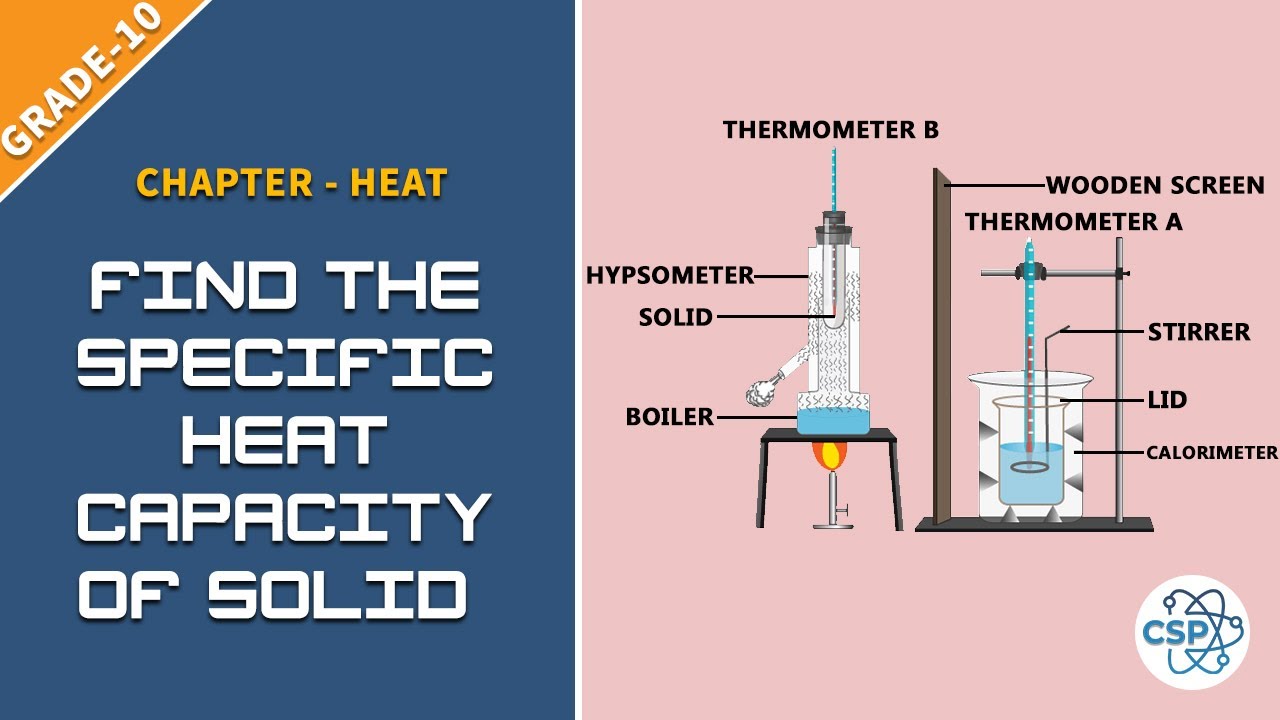
The specific heat capacity is different for different materials. The specific heat of a substance is dependent on both its molecular structure and its phase. Subtract the final and initial temperature to get the change in temperature ΔT. Find the final temperature of the mixture if two cup of water having masses m1150g and m2250g and temperatures T1 30 ºC and T275. Build Your Understanding - This is how to measure the specific heat capacity of a metal block. Change in thermal energy mass specific heat capacity.
 Source: pinterest.com
Source: pinterest.com
Specific Heat Capacity Unit Heat capacity Specific heat x mass. The heat capacity of a mixture can be calculated using the rule of mixtures. The specific heat capacity is different for different materials. 02 July 2021 If you want to update the article please loginregister. While we will often use heat capacity heat capacities are similar to mass that is their value.
 Source: pinterest.com
Source: pinterest.com
Or Cv for instance to denote the conditions under which the heat capacity has been determined. In this specified practical activity it is important to. The amount of thermal energy stored or released as the temperature of a system changes can be calculated using the equation. Or Cv for instance to denote the conditions under which the heat capacity has been determined. The formula for heat capacity is below.
 Source: pinterest.com
Source: pinterest.com
While we will often use heat capacity heat capacities are similar to mass that is their value. Energy mass specific heat capacity temperature change E m c θ. Specific Heat Capacity is the amount of heat required to make one kilogram of a substance one degree hotter. How to Calculate Specific Heat Capacity. Specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius.
 Source: id.pinterest.com
Source: id.pinterest.com
Find the initial and final temperature as well as the mass of the sample and energy supplied. In this video you will be introduced to specific heat capacity and you will learn how to solve heat capacity problems. Change in thermal energy Q is measured in joules J mass m is measured in kilograms kg specific. However you may not need it in the case of typical specific heat values. That is C Q 4T.
 Source: pinterest.com
Source: pinterest.com
Divide the heat. Multiply the change in temperature with the mass of the sample. C specific heat capacity for water in JkgC which is 41813 im not sure what error I would include here as its an pre-determined value off the internet So now I think I can. Find the initial and final temperature as well as the mass of the sample and energy supplied. The formula for heat capacity is below.
 Source: tr.pinterest.com
Source: tr.pinterest.com
Find the initial and final temperature as well as the mass of the sample and energy supplied. The new heat capacity depends on the. M 125 gm. The specific heat of a substance is dependent on both its molecular structure and its phase. Find the initial and final temperature as well as the mass of the sample and energy supplied.
 Source: in.pinterest.com
Source: in.pinterest.com
Specific Heat Capacity calculator uses specific_heat_capacity Energy Required Mass Rise in Temperature to calculate the Specific Heat Capacity Specific Heat Capacity is the heat required to raise the temperature of the unit mass of a given substance by a given amount. The specific heat capacity is different for different materials. Subtract the final and initial temperature to get the change in temperature ΔT. Change in thermal energy mass specific latent heat Q ml This is when. Specific Heat Capacity Unit Heat capacity Specific heat x mass.
 Source: pinterest.com
Source: pinterest.com
M 125 gm. Subtract the final and initial temperature to get the change in temperature ΔT. Multiply the change in temperature with the mass of the sample. Q m c θ where Q quantity of heat J. M mass kg.
 Source: pinterest.com
Source: pinterest.com
C specific heat capacity for water in JkgC which is 41813 im not sure what error I would include here as its an pre-determined value off the internet So now I think I can. The specific heat of a substance is dependent on both its molecular structure and its phase. Specific heat is the amount of heat per unit mass required to raise the temperature by one degree Celsius. The specific heat capacity c is the heat energy that is needed to raise the temperature of 1kg of the substance by 1 circ C. Specific Heat Capacity is denoted by c symbol.
 Source: pinterest.com
Source: pinterest.com
Find the initial and final temperature as well as the mass of the sample and energy supplied. The specific heat of a substance is dependent on both its molecular structure and its phase. The new heat capacity depends on the. Divide the heat. Specific Heat Capacity Formula Q C m t Where Q quantity of heat absorbed by a body m mass of the body t Rise in temperature C Specific heat capacity of a substance depends on the nature of the material of the substance.
 Source: in.pinterest.com
Source: in.pinterest.com
The specific heat of a substance is dependent on both its molecular structure and its phase. The specific heat capacity c. A piece of copper 125g has a heat capacity of 196876J also it is heated from 150 to 250 0 C heat. Multiply the change in temperature with the mass of the sample. C specific heat capacity for water in JkgC which is 41813 im not sure what error I would include here as its an pre-determined value off the internet So now I think I can.
 Source: in.pinterest.com
Source: in.pinterest.com
Find the initial and final temperature as well as the mass of the sample and energy supplied. Specific heat capacity in terms of heat capacity is conveyed as Problem 1. Heat Capacity The heat capacity of an object is the energy transfer by heating per unit tem-perature change. Change in thermal energy mass specific heat capacity temperature change Delta E_ t m times c times Delta theta This is when. Energy mass specific heat capacity temperature change E m c θ.
 Source: pinterest.com
Source: pinterest.com
Change in thermal energy mass specific heat capacity temperature change Delta E_ t m times c times Delta theta This is when. To know more examples and practice questions on Specific Heat Capacity. Change in thermal energy mass specific heat capacity temperature change Delta E_ t m times c times Delta theta This is when. The amount of heat given is equal to the amount of heat taken. C 196876125100 c 1575 Jg 0 C.
 Source: pinterest.com
Source: pinterest.com
Change in thermal energy mass specific heat capacity. Specific Heat Capacity Formula Q C m t Where Q quantity of heat absorbed by a body m mass of the body t Rise in temperature C Specific heat capacity of a substance depends on the nature of the material of the substance. C Q m x ΔT Q supplied or subtracted heat in joules M the samples mass ΔT initial and final temperature difference JkgK heat capacity measurement type Specific Heat Values The heat capacity calculator is going to be useful in many situations. In this expression we will frequently put subscripts on C Cp. Find out the specific heat.
 Source: in.pinterest.com
Source: in.pinterest.com
The new heat capacity depends on the. Change in thermal energy mass specific heat capacity. M 125 gm. Or Cv for instance to denote the conditions under which the heat capacity has been determined. In this specified practical activity it is important to.
 Source: pinterest.com
Source: pinterest.com
Specific Heat Capacity Formula Q C m t Where Q quantity of heat absorbed by a body m mass of the body t Rise in temperature C Specific heat capacity of a substance depends on the nature of the material of the substance. In this specified practical activity it is important to. ΔT 250-150 100 0 C. Change in thermal energy mass specific heat capacity temperature change Delta E_ t m times c times Delta theta This is when. M mass kg.
 Source: pinterest.com
Source: pinterest.com
The specific heat capacity c. Specific Heat Capacity Unit Heat capacity Specific heat x mass. That is C Q 4T. Heat Capacity The heat capacity of an object is the energy transfer by heating per unit tem-perature change. Object one has mass m1 temperature t1 and specific heat capacity c1 object two has mass m2 temperature t2 and specific heat capacity c2.
 Source: pinterest.com
Source: pinterest.com
Specific Heat Capacity is denoted by c symbol. In this specified practical activity it is important to. Specific Heat Capacity is denoted by c symbol. M mass kg. 02 July 2021 If you want to update the article please loginregister.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site serviceableness, please support us by sharing this posts to your own social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title how to work out specific heat capacity by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






