Your How to work out percentage uncertainty images are available. How to work out percentage uncertainty are a topic that is being searched for and liked by netizens now. You can Get the How to work out percentage uncertainty files here. Find and Download all free photos.
If you’re searching for how to work out percentage uncertainty pictures information linked to the how to work out percentage uncertainty topic, you have pay a visit to the ideal site. Our website frequently gives you suggestions for seeing the maximum quality video and image content, please kindly search and find more enlightening video content and images that fit your interests.
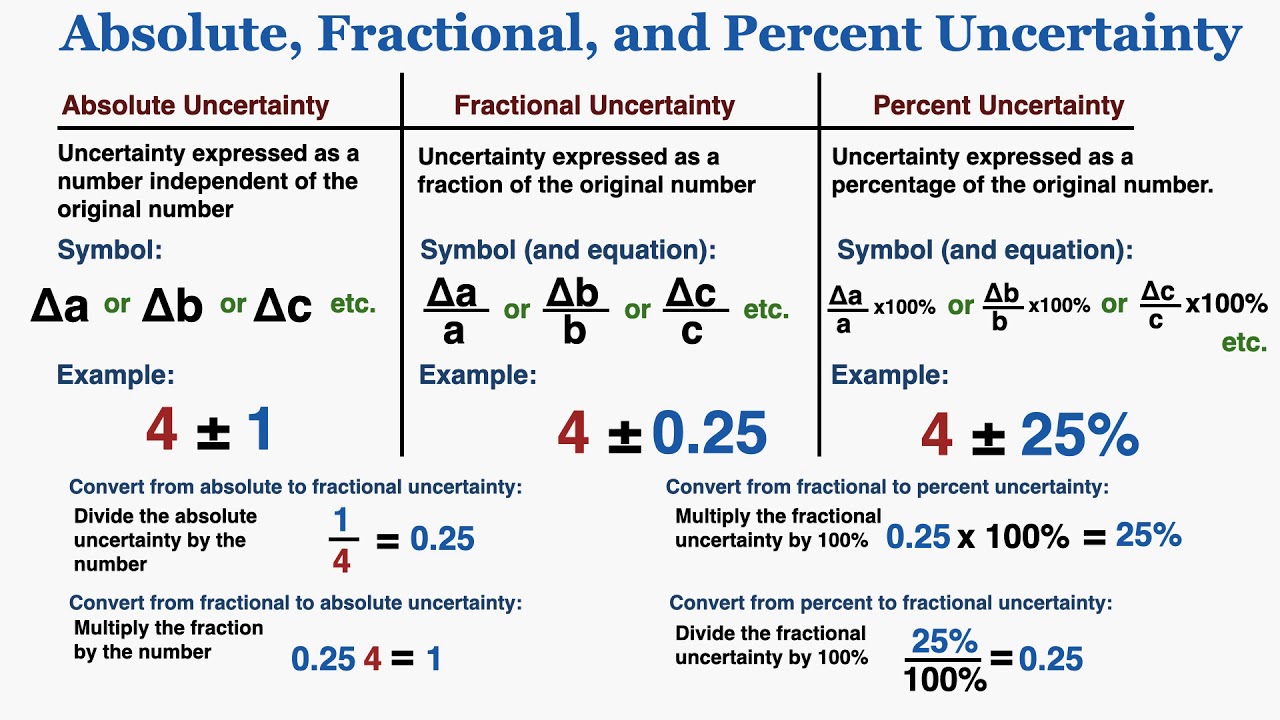
How To Work Out Percentage Uncertainty. For a thermometer with a mark at every 10C the uncertainty is. Uncertainty 015average titre result x100 To calculate the maximum total percentage apparatus uncertainty in the final result add all the individual equipment uncertainties together. Calculating Percentage Uncertainties when there are NO repeat measurements Reading on meter Resolution 126 V 02 V Uncertainty 01126 x 100 08 Uncertainty HALF Resolution x 100 Reading Taken Uncertainty HALF the Resolution 01V. Percentage uncertainty A percentage by definition is a value out of a potential hundred.
 11 1 State Uncertainties As Absolute And Percentage Uncertainties Sl Ib Chemistry Youtube From youtube.com
11 1 State Uncertainties As Absolute And Percentage Uncertainties Sl Ib Chemistry Youtube From youtube.com
Uncertainty 015average titre result x100 To calculate the maximum total percentage apparatus uncertainty in the final result add all the individual equipment uncertainties together. Explaining the difference between absolute uncertainty relative uncertainty and percentage uncertainty. For a thermometer with a mark at every 10C the uncertainty is. Work this out with. A look at the uncertainties associated with standard laboratory apparatus. Double-click an error bar in the chart to open the Format Error Bars pane.
Percentage Uncertainty Absolute Uncertainty Measured Value x 100.
Sometimes you may want to evaluate resolution uncertainty as a half digit. Percentage uncertainty in the y-intercept can also be found using the same method with the. Uncertainty 015average titre result x100 To calculate the maximum total percentage apparatus uncertainty in the final result add all the individual equipment uncertainties together. Percentage uncertainty best gradientworst gradient 100 The modulus bars in this equation mean that this value will always be positive. Work this out with. Uncertainty is calculated using the formula given below Uncertainty u xi μ2 n n-1 Uncertainty 003 seconds 68 of values fall within 1 standard deviation of the mean -1s.
 Source: slideplayer.com
Source: slideplayer.com
You can then adjust the percentage standard deviation value or even select a custom value from a cell that may have been produced by a statistical formula. Finally the expanded uncertainty U of the concentration of your standard solution is U k u_combined 12 in general k2 is used. Explaining the difference between absolute uncertainty relative uncertainty and percentage uncertainty. Three 10 gram weights are measured at 105 grams 100 grams and 095 grams. Fortunately there is a special notation for the percent uncertainty so it.
 Source: webassign.net
Source: webassign.net
How do you calculate percent uncertainty in chemistry. Worked examples of percentage uncertainty calculations and how to reduce them. Percentage uncertainty best gradientworst gradient 100 The modulus bars in this equation mean that this value will always be positive. 06 and we multiply it by 100 to get our percentage uncertainty. This is then multiplied by one hundred.
 Source: isobudgets.com
Source: isobudgets.com
Calculating Percentage Uncertainties when there are NO repeat measurements Reading on meter Resolution 126 V 02 V Uncertainty 01126 x 100 08 Uncertainty HALF Resolution x 100 Reading Taken Uncertainty HALF the Resolution 01V. TextRelative uncertainty fractextabsolute uncertaintytextbest estimate 100. -note the mass has been measured twice. 06 and we multiply it by 100 to get our percentage uncertainty. Work this out with.
 Source: nagwa.com
Source: nagwa.com
This is the just the relative uncertainty multiplied by 100. 35 J 31 J 04 J divide it by 2 and get an absolute uncertainty of 02 J. The percentage is calculated by taking the absolute error in a measurement and dividing by the value of the measurement itself. 06 and we multiply it by 100 to get our percentage uncertainty. Work this out with.
 Source: youtube.com
Source: youtube.com
A we must say that there has been some systematic error. Percentage uncertainty Uncertainty of measurementMeasurement 100 In the above example the percentage uncertainty in the diameter of the metal canister is. You will find that the result is the same. Percentage uncertainty best gradientworst gradient 100 The modulus bars in this equation mean that this value will always be positive. Work this out with.
 Source: sites.google.com
Source: sites.google.com
Since the percent uncertainty is also a ratio of similar quantities it also has no units. Explaining the difference between absolute uncertainty relative uncertainty and percentage uncertainty. A single reading cannot have a percentage uncertainty but a measured value. The percentage uncertainty in a measurement can be calculated using. A look at the uncertainties associated with standard laboratory apparatus.
 Source: youtube.com
Source: youtube.com
Worked examples of percentage uncertainty calculations and how to reduce them. You will find that the result is the same. How do you calculate percent uncertainty in chemistry. Worked examples of percentage uncertainty calculations and how to reduce them. -note the mass has been measured twice.
 Source: slideplayer.com
Source: slideplayer.com
Work this out with. Rather than divide the resolution by 2 and then by the square root of 3 you can convert it to a standard deviation equivalent by dividing resolution by the square root of 12. Explaining the difference between absolute uncertainty relative uncertainty and percentage uncertainty. Now we can write our value for work as W33 02 J. Percentage uncertainty in volume percentage uncertainty in L percentage uncertainty in L percentage uncertainty in L 31 31 31 93 But another way to write this is using the power p 3 times the uncertainty in the length.
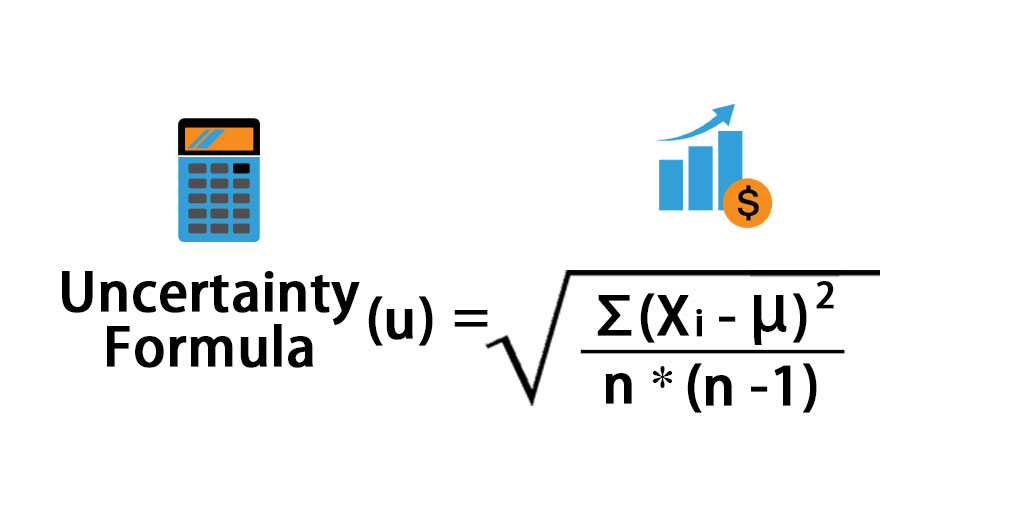 Source: educba.com
Source: educba.com
How do you calculate percent uncertainty in chemistry. A we must say that there has been some systematic error. Worked examples of percentage uncertainty calculations and how to reduce them. A look at the uncertainties associated with standard laboratory apparatus. Examples of Relative Uncertainty Calculations Example 1.
 Source: slideplayer.com
Source: slideplayer.com
If you are unfamiliar with this method give it a try. The percentage uncertainty in a measurement can be calculated using. 06 and we multiply it by 100 to get our percentage uncertainty. A look at the uncertainties associated with standard laboratory apparatus. Calculating Percentage Uncertainties when there are NO repeat measurements Reading on meter Resolution 126 V 02 V Uncertainty 01126 x 100 08 Uncertainty HALF Resolution x 100 Reading Taken Uncertainty HALF the Resolution 01V.
 Source: sites.google.com
Source: sites.google.com
TextRelative uncertainty fractextabsolute uncertaintytextbest estimate 100. Percentage uncertainty A percentage by definition is a value out of a potential hundred. Then you can work out the percentage uncertainty as. -note the mass has been measured twice. The relative uncertainty gives the uncertainty as a percentage of the original value.
 Source: mrsphysics.co.uk
Source: mrsphysics.co.uk
Finally the expanded uncertainty U of the concentration of your standard solution is U k u_combined 12 in general k2 is used. For burette 005 237 x 100 021show more. Collect Information and Data Evaluate and Select the Right Data Analyze the Data Quantify Uncertainty Components Collect Information and Data To get started you need to collect information and data related to your uncertainty analysis. To quantify uncertainty you need to follow the four steps below. Three 10 gram weights are measured at 105 grams 100 grams and 095 grams.
 Source: youtube.com
Source: youtube.com
Then you can work out the percentage uncertainty as. The percentage is calculated by taking the absolute error in a measurement and dividing by the value of the measurement itself. To quantify uncertainty you need to follow the four steps below. Uncertainty is calculated using the formula given below Uncertainty u xi μ2 n n-1 Uncertainty 003 seconds 68 of values fall within 1 standard deviation of the mean -1s. A single reading cannot have a percentage uncertainty but a measured value.
 Source: slideplayer.com
Source: slideplayer.com
Sometimes you may want to evaluate resolution uncertainty as a half digit. You can then adjust the percentage standard deviation value or even select a custom value from a cell that may have been produced by a statistical formula. To quantify uncertainty you need to follow the four steps below. Since the percent uncertainty is also a ratio of similar quantities it also has no units. Fortunately there is a special notation for the percent uncertainty so it.
 Source: youtube.com
Source: youtube.com
Then you can work out the percentage uncertainty as. The percentage uncertainty in a measurement can be calculated using. You will find that the result is the same. Percentage uncertainty 364 100. For pipette 006 25 x 100 024.
 Source: youtube.com
Source: youtube.com
A single reading cannot have a percentage uncertainty but a measured value. Explaining the difference between absolute uncertainty relative uncertainty and percentage uncertainty. 35 J 31 J 04 J divide it by 2 and get an absolute uncertainty of 02 J. Percentage uncertainty Uncertainty of measurementMeasurement 100 In the above example the percentage uncertainty in the diameter of the metal canister is. For a thermometer with a mark at every 10C the uncertainty is.
 Source: youtube.com
Source: youtube.com
For pipette 006 25 x 100 024. Rather than divide the resolution by 2 and then by the square root of 3 you can convert it to a standard deviation equivalent by dividing resolution by the square root of 12. If you are unfamiliar with this method give it a try. Double-click an error bar in the chart to open the Format Error Bars pane. The molality is the amount of substance in moles of solute the standard compound divided by the mass in kg of the solvent.
 Source: savemyexams.co.uk
Source: savemyexams.co.uk
A look at the uncertainties associated with standard laboratory apparatus. Percentage uncertainty Uncertainty of measurementMeasurement 100 In the above example the percentage uncertainty in the diameter of the metal canister is. To quantify uncertainty you need to follow the four steps below. Three 10 gram weights are measured at 105 grams 100 grams and 095 grams. Examples of Relative Uncertainty Calculations Example 1.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title how to work out percentage uncertainty by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.